THE-01: Thermal Expansion of Solid
The linear expansion coefficient α is determined by the material of the solid body. We can conduct measurements on this topic using e.g. thin tubes through which hot water or steam flows.

Experiment:
| THE-01-01 | Thermal expansion of solids – Measuring using the expansion apparatus | |||||
THE-02: Thermal Conductivity
In the equilibrium state, the heat flow through a plate with the cross-section area A and the thickness d depends on the temperature difference ϑ2 – ϑ1 between the front and rear sides and on the thermal conductivity λ of the plate material

Experiments:
| THE-02-01 | Determining the thermal conductivity of building materials using the single-plate method | |||||
| THE-02-02 | Determining the thermal conductivity of building materials using the heat flux plate principle | |||||
| THE-02-03 | Damping temperature fluctuations using multiple-layered walls | |||||
THE-03: Solar Collector
A solar collector absorbs radiant energy to heat the water flowing through it. When the collector is warmer than its surroundings, it loses heat to its surroundings through radiation, convection and heat conductivity.

Experiments:
| THE-03-01 | Determining the efficiency of a solar collector as a function of the throughput volume of water | |||||
| THE-03-02 | Determining the efficiency of a solar collector as a function of the heat insulation | |||||
THE-04: Converting Electrical Energy into Heat
The equivalence of electrical energy Eel and thermal energy Eth is established experimentally. The supplied electrical energy Eel is converted into heat Eth in the heating coil (or heating spiral). This leads to a temperature rise in the calorimeter (or water, in which the heating spiral is immersed). As the current I and the temperature ϑ are measured simultaneously as functions of the time t, the constant voltage U being known, the two energy forms can be registered quantitatively in units of wattsecond (Ws) and Joule (J) so that their numerical equivalence can be demonstrated experimentally:
Eel = Eth.

Experiment:
| THE-04-01 | Converting electrical energy into heat energy – Measuring with CASSY | |||||
THE-05: Gas Laws
The gas thermometer consists of a glass tube closed at the bottom end, in which a mercury stopper seals the captured air at the top. The volume of the air column is determined from its height and the cross-section of the glass tube. When the pressure at the open end is altered using a hand pump, this changes the pressure on the sealed side correspondingly. The temperature of the entire gas thermometer can be varied using a water bath.
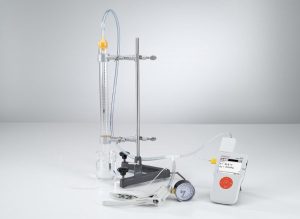
Experiments:
THE-06: Hot Air Engine (Quantitative Experiment)
When the hot-air engine is operated as a heat engine, each engine cycle withdraws the amount of heat Q1 from reservoir 1, generates the mechanical work W and transfers the difference Q2 = Q1 – W to reservoir 2. The hot-air engine can also be made to function as a refrigerating machine while operated in the same rotational direction by externally applying the mechanical work W. In both cases, the work WF converted into heat in each cycle through the friction of the piston in the cylinder must be taken into consideration.
Thermodynamic cycles are often described as a closed curve in a pV diagram (p: pressure, V: volume). The work added to or withdrawn from the system (depending on the direction of rotation) corresponds to the area enclosed by the curve.

Experiment:
| THE-06-01 | pV diagram of the hot-air engine as a heat engine – Recording and evaluating with CASSY | |||||
Video:
[embedyt] https://www.youtube.com/watch?v=XMwAnLUt7Zg[/embedyt]
